 |
 |
|
IntroductionAs long as ancient coins have been collected numismatists have debated whether ancient coins were struck hot or cold. On seeing blacksmiths hammer glowing red iron into useful objects, many assume gold, silver or copper behave the same way so such coins would also have been struck while heated. That assumption is incorrect and I have written this website to explain why. I will keep this as simple as the subject allows, while providing links to more detailed discussions for those who wish to read further. DefinitionsA few terms that need defining: Unit Cell Structures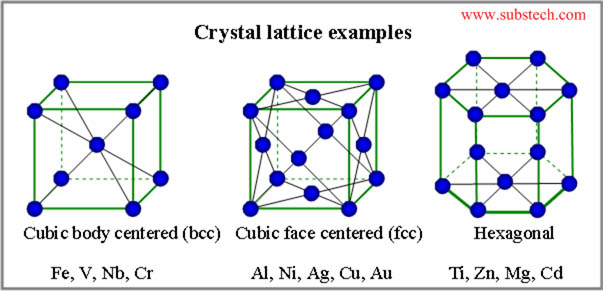 Image from Subtech.com, under creative commons Body Centered Cubic (BCC) metals have unit cells of nine atoms. One atom in the center bonded to eight atoms forming a cube around it. Two BCC unit cells join to form a crystal by sharing the four atoms of a cube face with that face bonded to the center atoms of both cells. In larger structures each unit cell shares all six faces with adjoining cells and will be bonded to its own center atom and all six center atoms of the cells around it. This is a strong structure of low malleability. Face Centered Cubic (FCC) metals form unit cells of fourteen atoms, eight at the corners of a cube with the other six on the cube faces, like numbers on a dice. Two FCC unit cells join by sharing the five atoms of a cube face, repeating on all six faces in larger crystals. With no atoms in the center of each cube, the faces are only weakly bonded with high malleability as the cell faces can slip and join other cells when under compression. The malleability of on FCC metal is same at all temperatures below melting point so an FCC medal does not soften when heated. Further reading on BCC and FCC crystal structuresFurther reading as to why FCC metals are more malleable than BCC metals Below 910 C iron is a BCC metal of low malleability but when heated to between 910 C and 1394 C the atoms shift to an FCC structure of higher malleability. This allows blacksmiths to hammer red hot iron into shapes which once cooled are again hard as the iron reverts back to a BCC structure. This property is known as allotropy. Further reading on iron conversion from BCC to FCC at high temperaturesGold, silver and copper are FCC metals and like all FCC metals the malleability is the same at all temperatures below the melting point. This means heating does not increase malleability (softness) at higher temperatures in the way iron does. AlloysThe FCC properties described above are how pure gold, silver and copper behave. Ancient refining was imperfect with an upper limit usually around 98.5% pure for gold and silver. The remaining 1.5% is a mix of various impurities each of too low percentage to have significant effect on the FCC structure and properties. Many ancient coins were struck on this "pure" metal but others were intentionally alloyed, usually silver with copper, gold with silver and/or copper, and copper with tin and sometimes zinc. Copper is soluble in silver up to about 7%, meaning copper atoms can displace up to 7% of the silver atoms in a silver unit cell. Silver is also soluble in copper at about 7% in the same way. Silver mixed with more than 7% copper will first dissolve 7% copper into the silver unit cells which form crystals and grains of silver. Excess copper forms copper unit cells dissolving 7% silver which form crystals and grains of copper. If there is more copper than silver only the percentage of silver and copper grains varies. The grain types disperse fairly evenly through the alloy. The exact malleability varies with the exact alloy but once formed all silver copper alloys are still FCC metals. Most ancient gold coins were relatively pure gold but some are alloyed with copper, silver or a mix of the two. Most alloys of gold with silver and/or copper are fully soluble in each other and all such alloys are FCC metals. Further reading about gold alloysThe malleability of copper alloyed with tin can vary considerably depending on the percentage of tin, how quickly the alloy was cooled, and if it was reheated after cooling. A discussion of copper-tin alloy malleability is complex but for the purpose of this discussion it is only important to understand all alloys of copper and tin, even if lead are zinc are added, will be FCC metals. Further reading about copper alloys, although mostly focusing on modern alloys rather than ancient.As Gold, silver and copper alloys are all FCC metals; their malleability is not affected by temperature. Work hardening/AnnealingAs metals deform under compression dislocations can occur in the crystal structures resulting in work hardening where malleability is reduced and the metal becomes more fragile. Annealing is a process where the metal is heated for a few minutes to a temperature at which the atoms can realign to their original unit cells, eliminating dislocations thus returning to original malleability. Annealed FCC metals can then be cooled to room temperature with no decrease in malleability and there will be increase at higher temperatures. Cast gold, silver or copper (and their alloy) coin blanks are naturally annealed and can be struck as is. Blanks hammering flat will be work hardened and must be annealed before they can then be struck at room temperature. Further reading about work hardeningFurther reading about annealing Experiments In Cold Striking.Many people have experimented with hot and cold hammer striking of high relief coins. Most fail to produce satisfactory quality coins either hot or cold, but a few have demonstrated high relief tetradrachm sized silver coins can be successfully struck at room temperature. This video is titled "Badger Mint Hot Striking" but he took the blank flan from a bucket of flans with his unprotected fingers, which he could not have done is the flan was hot. I suspect "Hot Striking" they mean the flans had been heated to anneal them, not that the flans were struck while hot. This video leaves no doubt that the blanks are sitting in a pile and fully cool prior to striking. ConclusionGold, silver, copper and their alloys are FCC metals which once annealed are in their most malleable state at all temperatures. Striking while heated would not improve the ease of striking or the quality of the finished coins. Ancient people would not have understood the atomic structures involved but had been working with these metals for more than 1000 years by the time the first coins were struck in the mid-7th century BC. They would have understood how these metals behave and that striking on heated blanks would provide no advantage over striking at room temperature. 
Copyright © 2017 R & T Enterprises Ltd. |